עיתונאיות ועיתונאים, הירשמו כאן להודעות לעיתונות שלנו
הירשמו לניוזלטר החודשי שלנו:
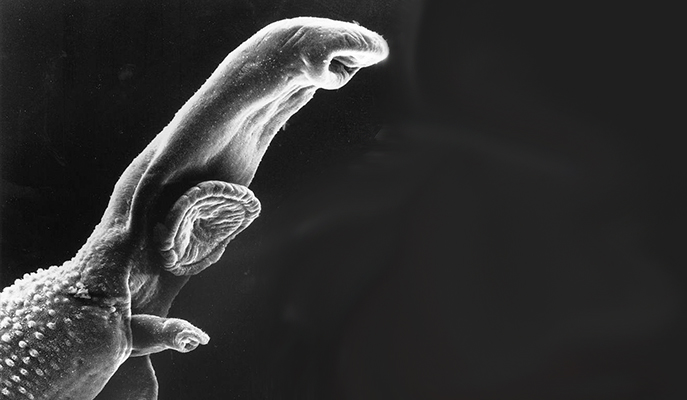
The current surge of allergies in the Western world might be the price we pay for being exposed to fewer infections. This is the so-called “hygiene hypothesis”: Exposure to infectious organisms somehow offers protection against allergies, so people living in a relatively sterile environment fail to benefit from this defense. New support for this hypothesis comes from a collaboration between researchers from the Weizmann Institute of Science, Sheba Medical Center and Bar-Ilan University. In a study published in EMBO Reports, they found a link between the parasitic worm that causes bilharzia – also known as snail fever, which affects more than 200 million people worldwide, mostly in Africa – and the sort of reduction in the immune response that produces allergic disorders.
The research project was born of a chance meeting between two Israeli researchers at a scientific conference in the United States. Dr. Neta Regev-Rudzki of Weizmann’s Biomolecular Sciences Department told Prof. Eli Schwartz of Sheba Medical Center about her discovery that the malaria parasite sends misleading messages to an infected person’s immune system by releasing small segments of its DNA encapsulated in sac-like “packages,” or nanovesicles. Schwartz, who had treated many travelers with tropical infections, wondered whether schistosomes, the bilharzia parasites, employ the same strategy. If so, this could help explain how the worm evades detection by the immune system, and it could have promising implications for the diagnosis and possible treatment of bilharzia.

People become infected with schistosomes while swimming in lakes or other freshwater reservoirs. The worms mature in the human body, producing thousands of eggs; some of these get into the water after being ejected in fecal matter or urine, hatch into larvae and infect freshwater snails, in which they develop further before being released back into the water, ready to infect more humans or other mammals. The infection sometimes causes pain, diarrhea and other symptoms, but it can also linger in the body for years without causing symptoms. Bilharzia is diagnosed by a blood test that detects antibodies against the worms, but since antibodies tend to persist even after the worms themselves have been killed by drugs, the test cannot distinguish between an active and a past disease. The researchers reasoned that if the worms use the malaria parasites’ trick – releasing nanovesicles – their presence might reveal live worms. In addition to providing a more precise diagnosis, finding such nanovesicles could help researchers clarify how the parasite affects the immune system of its human host.
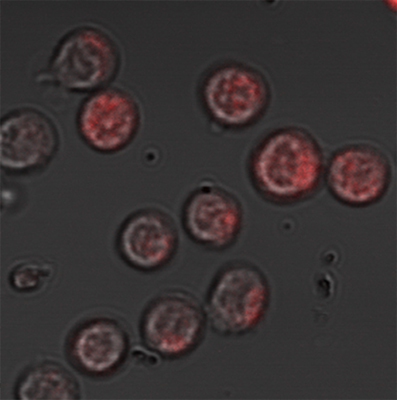
To conduct this research, Dr. Dror Avni and colleagues at Sheba Medical Center teamed up with Regev-Rudzki at Weizmann and with immunologist Dr. Orly Avni of Bar-Ilan University’s Azrieli Faculty of Medicine. After adapting the techniques Regev-Rudzki had developed in her malaria studies, the researchers used electron microscopy and atomic force microscopy to analyze particles isolated from a solution in which they’d grown adult worms. They found that, like the malaria parasites, the schistosomes release characteristic nanovesicles that contain small segments of their genetic material known as microRNAs. The researchers then found the same microRNA-nanovesicle packages in blood samples taken from people infected with bilharzia. The amounts of these microRNAs decreased significantly after treatment, suggesting that they had indeed been manufactured by live worms.

To explore the effects of the vesicles on the immune system, the researchers first confirmed that immune T cells isolated from lymph nodes of mice infected with bilharzia did, indeed, contain microRNA from the worms. The scientists then created a laboratory setup in which the worms were separated by a filter from a dish containing primary T cells – that is, T cells that had not yet differentiated into specific subtypes. The filter allowed the worms’ packages to pass through, but not the worms themselves. The scientists found that when the nano-packages penetrated the T cells, depositing the worms’ microRNAs inside these cells, the genetic material blocked biochemical reactions that are supposed to help the T cells differentiate into a subtype called Th2. Th2 is responsible for activating the branch of the immune system that fights parasitic infections. In other words, the worms dispatch a treacherous message in their nano-packages that prevents the immune system from responding to them.
These findings explain how the bilharzia infection can persist for years without provoking an immune response. The findings also provide support for the hygiene hypothesis: Th2 cells are the ones mainly responsible for asthma, atopic dermatitis and various other allergic and autoimmune disorders, in which the immune system mistakenly attacks its own tissues and organs. These diseases are uncommon in developing countries where the high prevalence of parasitic infections may suppress Th2 cells.
The study’s findings may lead to improved methods of diagnosing bilharzia and of following the progress of treatment that would be based on detecting the worm’s genetic material rather than antibodies. And future treatments might one day be developed to prevent the nano-packages from reaching the immune system, so that it can mount a proper defense against the parasites. In addition, when the interactions between the nano-packages and the immune system are better understood, it may be possible to develop, on the basis of these interactions, new ways of manipulating the immune system to help prevent or treat autoimmune disorders.
Study participants included Dr. Tal Meningher and Prof. Yechezkel Sidi from the Sheba Medical Center; Dr. Yiftah Barsheshet and Boris Brant from Bar-Ilan University’s Azrieli Faculty of Medicine; Prof. Daniel Gold from Tel-Aviv University and Dr. Yifat Ofir-Birin and Elya Dekel from Weizmann’s Biomolecular Sciences Department.
Dr. Neta Regev-Rudski's research is supported by the Benoziyo Endowment Fund for the Advancement of Science; the Dr. Dvora and Haim Teitelbaum Endowment Fund; the Weizmann – UK Making Connections Programme; Richard and Mica Hadar; and David E. and Sheri Stone.