עיתונאיות ועיתונאים, הירשמו כאן להודעות לעיתונות שלנו
הירשמו לניוזלטר החודשי שלנו:

How does a cell sense its own size? That question becomes especially significant when the cell is large: a human peripheral neuron, for example, which can grow extensions reaching up to a meter in length – some 20,000 times the cell body’s diameter. Without some basic information on the distance involved, just getting the building materials out to the growing end could be a logistical nightmare, exposing the cell to logjams in the supply line. These cells most probably assess their dimensions on an ongoing basis in order to direct further growth, but until now, no one was quite sure how they accomplished this feat.
Dr. Ida Rishal and Prof. Michael Fainzilber of the Weizmann Institute’s Biological Chemistry Department and their collaborators have now provided the answer for large cells such as neurons. Among other things, their findings may have relevance for understanding how to accelerate the repair of damaged nerves.
Fainzilber and Rishal, together with Dr. Naaman Kam and Rotem Ben-Tov Perry of the Biological Chemistry Department, Dr. Vera Shinder of the Chemical Research Support Department, Prof. Elizabeth Fisher of University College London and Prof. Giampietro Schiavo of Cancer Resesarch UK London Research Institute, thought the solution might lie in the motorized transport system that runs up and down the length of the nerve cell. This system consists of tracks called microtubules and two families of motors known as kinesin and dynein. One, the kinesin, travels only from the cell center to the end of the extension; the other, the dynein, goes only in the other direction, toward the center. Could these two, in addition to hauling cargo of various sorts up and down the tracks, be measuring distance?
The researchers began by constructing computer models of possible mechanisms by which these cellular motors might measure length. In the first model, dynein motors moving toward the cell center would carry signals that would gradually be lost along the way, like breadcrumbs dropping at a constant rate. The amount of signal left at the end of their journey would reveal the distance traveled. In this model, if the number of dynein motors was smaller than normal, the lowered signal would make the cell seem larger than it is, and thus the extensions would grow more slowly and end up shorter.
A second proposed mechanism involved a feedback loop between the two motors. When the signal on one motor reached the end of the line, it would activate a signal on the second, sending it back in the other direction. The second signal would then terminate the activity of the first at the opposite endpoint. The measurement in this case would be based on timing – more specifically, on the frequency of the interactions at the terminal points. This frequency is something like a game of Ping-Pong: When played in a small area close to the net, the “pings” are rapid; bounces off the table’s far edges, by contrast, produce longer pauses between hits. As opposed to the first model, this one predicted that reducing the levels of either motor would actually result in faster-growing, longer nerve cell extensions, because the signal frequency would drop more slowly.
The team then conducted experiments, first in nerve cells grown in culture and then in mice, in which the levels of the dynein motor were reduced. In all instances, the extensions grew longer than usual, ruling out the first model and supporting the second one.
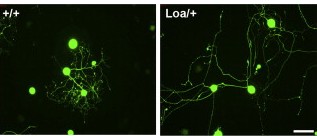
Finally, the team asked whether this mechanism is specific to nerve cells, or whether other large cell types might use similar methods to sense their size. Repeating their experiment in connective tissue cells called fibroblasts, they again found evidence for motor-based frequency signals.
In addition to providing the answer to a long-standing question about size-sensing in large cells, these findings may be relevant to research on the regeneration of nerve cells. Cells in the peripheral nervous system can grow back after injury, but the process is extremely slow – sometimes taking years in the case of the longest nerve extensions. This is in part because once a growing nerve cell connects with its target – usually sometime during embryonic development – it stops elongating from the end and, instead, grows along with the body by stretching throughout its length. Understanding the precise signals the cell uses to not only measure itself but then to direct the embryonic growth process accordingly might point to new directions in boosting nerve regeneration.
Prof. Michael Fainzilber's research is supported by the Sylvia Schaefer Alzheimer's Research Fund; the Kahn Family Research Center for Systems Biology of the Human Cell; the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; the Legacy Heritage Fund Program of the Israel Science Foundation; the Nella and Leon Benoziyo Center for Neurological Diseases; the Yeda-Sela Center for Basic Research; the estate of Raymond Lapon; the Irwin Green Alzheimer's Research Fund; and the estate of Florence Cuevas. Prof. Fainzilber is the incumbent of the Chaya Professorial Chair in Molecular Neuroscience.