One of the ways our cells keep things running smoothly is by recycling all sorts of proteins, especially those that are damaged or have outlived their usefulness. But how does the cell avoid consigning to the recycling bin those proteins that are still serving important functions? New research led by
Dr. Michal Sharon of the Biological Chemistry Department reveals how one enzyme works to rescue vital proteins from unwanted demolition. Among other things, this enzyme saves from the cell’s protein recycler two proteins that help stop cancerous growth – p53 and p73 – and thus may play a role in protecting against cancer.
Several years ago,
Prof. Yosef Shaul, Head of the Molecular Genetics Department, revealed a part of the answer: He identified the “rescuer” that prevents unwanted dismantling. This rescuer, an enzyme called NQO1, is an all-around protector of the cell: In addition to keeping vital proteins from being broken down, it was known to fight against reactive oxygen compounds, thus protecting the cell from oxidative damage.
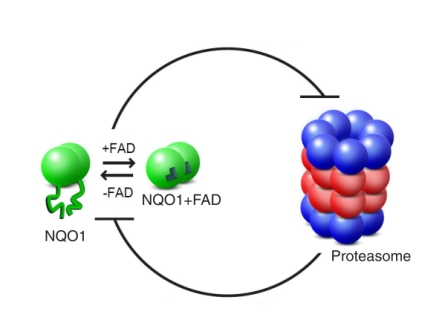
To investigate how NQO1 saves proteins from dismantling, Oren Moscovitz, Nimrod Hazan, Hodaya Keisar, and Drs. Gili Ben-Nissan and Izhak Michaelevsky in Sharon’s group created a simplified version of the recycling process. Into their experimental system went enzymes and the recycling unit of the proteasome. By leaving out the first proteasome unit – the one that checks the ubiquitin tags – they were assured of observing just the passive dismantling pathway. Next, the researchers added another molecule – one derived from vitamin B2 called FAD. The binding of FAD molecules to NQO1 is necessary for it to carry out its cellular duties.
Observing the NQO1 enzyme using the advanced mass spectrometry equipment in Sharon’s lab, the team discovered that the bound FAD molecules serve to stabilize the enzyme’s structure. They then tested this observation with a mutant NQO1 enzyme in which the FAD binding site is defective, as well as checking what happened when they removed FAD from a normal molecule. In both cases, the result was a shapeless, unfolded enzyme that disappeared from the experimental system, as it was dismantled in the proteasome grinder unit. When they added large quantities of FAD to the system, the enzymes – both normal and mutant – resumed their organized shapes and were saved from demolition.
Together with Shaul and his research student Peter Tsvetkov, the team tested their findings in living cells. They found that giving vitamin B2 to these cells not only increased NQO1 levels and stabilized its structure, it also boosted p53 levels. Further testing on breast cancer cells in which FAD binding was faulty added to the picture: Giving these cells B2 rescued the NQO1 that would have been dismantled, and it, in turn, saved the cells’ p53. Since p53 is so important for preventing cancer, says Moscovitz, “we think of this as ‘to B2 or not to be.’”
The findings, which appeared recently in Molecular Cell, show that the relationship between the proteasome and NQO1 is based on a sort of mutual deterrence. The proteasome does, indeed, dismantle NQO1 enzymes that lack a complete structure, while enzymes with stable structures can block the actions of the proteasome, rescuing other proteins in the process. The factor that tips the scales is vitamin B2, a nutrient that is absorbed from outside the body. The scientists think that the phenomenon they have discovered – a metabolic factor that directs the functioning of a system by affecting the shape of one of its components – may be found in other cellular pathways, as well.
The mutation that obstructs NQO1 binding to FAD is found in around 4% of the human population and a fifth of all Asians. The cells of people with this mutation do not cope well with oxidative stress, and they contain particularly low levels of p53 and p73. These people tend to be susceptible to cancer – especially breast cancer and leukemia. Thus the findings, which suggest that large quantities of vitamin B2 might overcome this problem, hint at a possible new and important role for this nutrient.
Dr. Michal Sharon’s research is supported by the Wolfson Family Charitable Trust; and Karen Siem, UK. Dr. Sharon is the incumbent of the Elaine Blond Career Development Chair in Perpetuity.
Prof. Yosef Shaul’s research is supported by the M.D. Moross Institute for Cancer Research; the Leo and Julia Forchheimer Center for Molecular Genetics, which he heads; the Leona M. and Harry B. Helmsley Charitable Trust; the Ben May Charitable Trust; and the Cure Foundation. Prof. Shaul is the incumbent of the Oscar and Emma Getz Professorial Chair.