A baby with dangerously low levels of certain white blood cells recovers, while an adult with nearly twice the levels succumbs to bacterial infection. A new mathematical model proposes a possible explanation for such medical mysteries, shedding light on those appearing after chemotherapy, and even suggesting how their occurrence could be reduced.
This study, reported recently in the
Journal of Clinical Investigation, has shown that to properly assess the risk of infection, it is essential to evaluate not only the quantity of immune cells but also their quality, which varies from one person to another. This research may therefore lead to more personalized chemotherapy: Better precautions might be taken to prevent infection in high-risk patients, whereas those at a low risk could be spared unnecessary preventive treatments. The new model was developed by Weizmann Institute mathematicians in collaboration with physicians from the Meir Medical Center in Kfar Saba and from the Hoffmann-La Roche research center in Basel, Switzerland.
The model reveals how the immune system functions under conditions of neutropenia – a dangerously low level of white blood cells, mainly neutrophils. In this condition, which often emerges after chemotherapy or bone marrow transplant but can also be present at birth, severe infections can develop if the immune system fails to perform the crucial function of devouring and destroying bacteria.
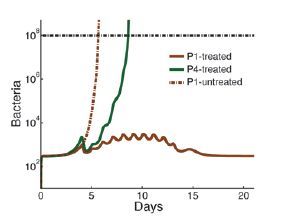
The tug of war between the blood cells and the bacteria cannot be explained away by the simple bacteria-to-cell ratio, nor by a threshold that the blood cell count must exceed. Rather, the model shows that when neutrophil counts are low, the patient’s immune system enters a fragile equilibrium – described in mathematical terms as “bistability” – that can easily be disrupted, with dramatic consequences, by even minute changes in bacterial concentration or in the number of neutrophils. Other factors that can dramatically affect this equilibrium include the effectiveness of the neutrophil functioning and the tissues’ permeability to bacteria, which can increase due to cancer therapy.
Thus, according to the model, in healthy people, the fact that the effectiveness of neutrophils varies from one person to another usually has no significance. By contrast, in patients with neutropenia, this individual variability can make a difference between life and death. For example, after chemotherapy, some cancer patients contract life-threatening infections even when they are maintained in isolation under sterile conditions. It turns out that if the neutrophils of these patients are "weak," even the smallest number of bacteria, such as those present in the gut, can tilt the fragile immune balance in favor of the bacteria.
The study also explains why certain patients following chemotherapy or a bone marrow transplant may develop an acute infection even when their neutrophil levels have returned to relatively normal levels. The chemotherapy lowers the neutrophil levels and function, in addition to making the tissues of these patients more penetrable to bacteria. As a result, in some patients the bacterial concentrations might increase so quickly that by the time the neutrophil counts rise back to “normal,” the rapidly multiplying bacteria have already gained a head start, so that the neutrophil recovery is insufficient for overcoming the severe infection. This scenario may eventually also shed light on the rare cases in which acute bacterial infections develop in individuals with normal immunological function. The model suggests that in such cases, a high growth rate of unusually virulent bacteria may have overcome even the appropriate quantitative and qualitative neutrophil response.
A potential solution to two previously unsolved cases has already emerged from the model. A newborn baby treated at Meir Medical Center recovered from neutropenia even though his absolute neutrophil count (ANC) had fallen as low as 200 neutrophils per microliter of blood, whereas an adult whose ANC stood at 380 after chemotherapy died of infection. The model has suggested how various clinical parameters, such as the poor quality of the neutrophils, could have led to the death of the adult despite his higher ANC.
In addition, the model might help researchers understand the mechanism behind the development of severe recurrent infections in some patients. For example, of one thousand patients referred to Meir Medical Center because of such infections, diagnosis could be established in only one-third of the cases. Weizmann Institute mathematical modeling showed that at least some of the unexplained cases might have resulted from a combination of several mild defects, including variation in the function of neutrophils and other immune cells.
This research was based upon the blood analysis of four healthy volunteers. To use the model in the clinic, such analysis will have to be applied to large populations. “Our mathematical model has revealed previously unknown mechanisms responsible for the variability in the vulnerability to infections of neutropenia patients,” says research leader
Prof. Vered Rom-Kedar of the Weizmann Institute’s Computer Science and Applied Mathematics Department.
The study was performed by researchers with an unusual combination of backgrounds. The mathematician, Rom-Kedar, specializes in the investigation of dynamic systems. The first author, electrical engineer Dr. Roy Malka, conducted this research as part of his Ph.D. studies in mathematics at Weizmann; he is now engaged in postdoctoral research on related subjects at Harvard Medical School. The idea for the project was first proposed by Dr. Eliezer Shochat, a senior oncologist who also has a Ph.D. in applied mathematics from Weizmann and now works in a research group at Hoffman-La Roche in Basel, Switzerland. The study was performed in collaboration with the Meir Medical Center team: Prof. Baruch Wolach, M.D., Head of the Laboratory for Leukocyte Function and Chair of Pediatric Immunology at Tel Aviv University’s Sackler Faculty of Medicine; and laboratory manager Ronit Gavrieli, M.Sc., who performed the experiments.
Says Prof. Wolach: “Our study suggests that to achieve optimal results – in applying chemotherapy and/or in treating patients with innate neutrophil dysfunction – it is of value to periodically assess the patient’s neutrophils as well as the bacterial concentration. Such assessments will help reduce the morbidity and mortality, as well as the cost, associated with unnecessary hospitalizations and the administration of expensive medications. Moreover, by cutting down on the use of antibiotics, these assessments can help prevent the rise in antibiotic resistance.”
Prof. Vered Rom Kedar heads the Moross Research School of Mathematics and Computer Science; her research is supported by the Yeda-Sela Center for Basic Research. Prof. Rom Kedar is the incumbent of the Estrin Family Chair of Computer Science and Applied Mathematics.