With modern maps, we can zoom out to a region’s entire road system or zoom in to distinguish the streets leading from one particular point to another. Somewhere in the middle of our zooming we might note the pattern of roads connecting neighboring cities that create interdependence between them. In the lab of
Prof. Adi Kimchi of the Molecular Genetics Department, researchers creating such a map for two “cities” in the cell are coming to understand just how important the interactions created by such connecting roads might be for our body’s cells. Among other things, this arrangement may help prevent the growth of cancer.
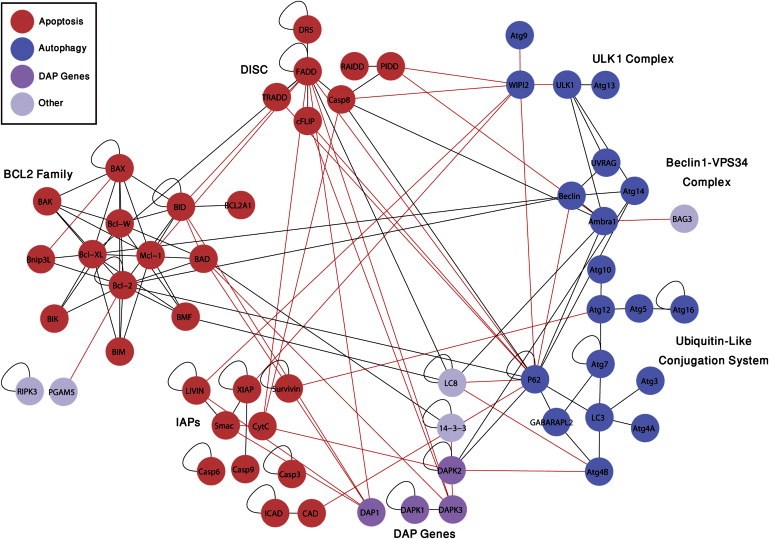
The “cities” are molecular pathways that end in two different types of cell death. A pathway, in the language of molecular biology, is the set of proteins and the series of events they mobilize that lead to a particular end. Such pathways may comprise large networks of proteins and complex, multi-stage communications systems. Hundreds of proteins in each cell death pathway must talk to one another to opt for, initiate and orchestrate the process.
Cell death is so important to the body that it has at least three different pathways for accomplishing this: apoptosis, autophagy and necrosis. As opposed to the latter, which is a violent death generally due to wounds or infection, apoptosis is a considered and planned rapid suicide. It often comes into play during development, when the embryo is constantly being remodeled, or later, when damage to the DNA of a cell threatens to turn it cancerous. Autophagy, unlike necrosis or apoptosis, does not always lead to cell death: It can be a survival mechanism, shutting down parts of the cell’s functioning in stressful conditions and recycling nonessential proteins for emergency use. But when needed, this same mechanism can also recycle the cell out of existence.
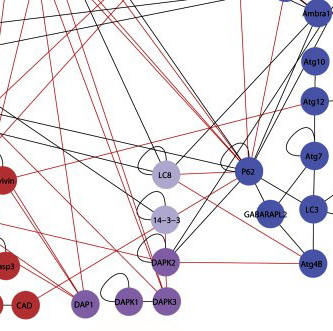
Until recently, researchers had thought that apoptosis and autophagy were like independent walled cities, each with its own separate system of roads. But recent research in Kimchi’s lab reveals a more modern landscape, in which the two pathways veer into one another’s territory, interconnected by crisscrossing trails.
To map out the region where the two pathways meet, Kimchi and research student Yuval Gilad created an experimental system to check for interactions between the proteins in both pathways. Gilad did this by attaching pieces of a protein called luciferase – the molecule that makes bioluminescent plankton glow – to the pathway proteins. In the experiment, the luciferase emitted light when two proteins with matching halves met, thus reporting on a connection between these pairs. Each connection then added another line to the map.
This new map was recently published in
Cell Reports.
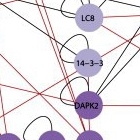
All together, after testing thousands of possible pairs, Kimchi and Gilad identified 46 new interactions, “each with a story to tell,” says Kimchi. Next, they zoomed in on one particular location on the map, a protein known as DAP kinase 2 (DAPK2). DAP kinases, a family of proteins discovered nearly two decades ago in Kimchi’s lab, play a prominent role in apoptosis. But the six lines on the map leading to and from DAPK2 suggested that it is active in both pathways. Zooming in further, the researchers looked closely at one of its connections – an interaction with a protein known as 14-3-3. The protein 14-3-3 binds to a unique region on DAPK2, preventing it from assuming its active form. The discovery of this interaction revealed a new layer of regulation on DAPK2 activity. Since it’s activation can lead to cell death, says Kimchi, it will be interesting to look for treatments that activate DAPK2 in cancer cells by inhibiting its interaction with 14-3-3.
The new map reveals just how complex the business of cell death can be. The researchers believe that the interconnections between the pathways provide the cell with a backup in case one of them fails. This is especially important when a cell that could be cancerous needs to self-destruct; the growth of cancer usually involves a failure of the cell death pathways. Kimchi and Gilad say that this map and the method they used to create it could provide a valuable tool to both cancer researchers and drug developers. Not only has it revealed a rich new landscape of protein interactions, it can expose the stages at which these interactions occur. Since cancer cells tend to evade death, drug designers might attempt to target specific stages of the cell death process in order to encourage the cells to “commit suicide.” The new map can help highlight the better targets for drug screening and the reporters detected in the study can be used as tools for the identification of such drugs.
Prof. Adi Kimchi's research is supported by the Helen and Martin Kimmel Institute for Stem Cell Research; the EMET Prize sponsored by the A.M.N. Foundation for the Advancement of Science, Art and Culture in Israel; and the estate of Katherine and Ladislaus Braun.