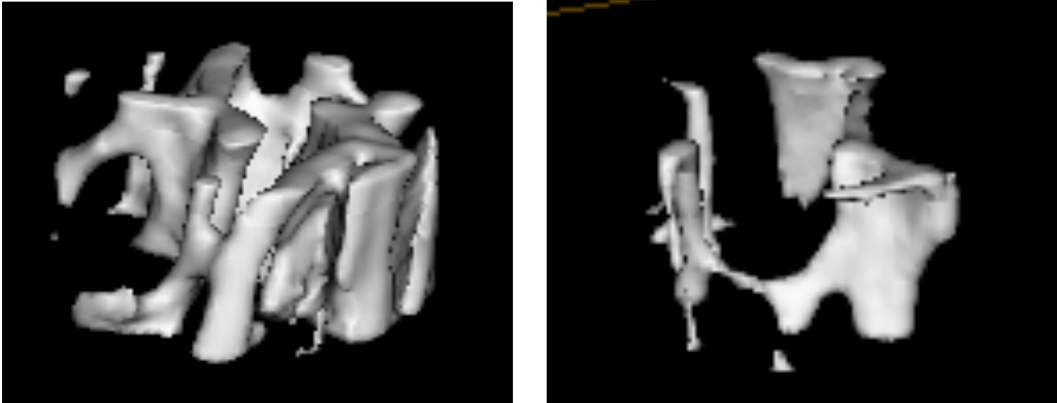
As reported recently in
eLife, they have now determined exactly how galectin-8 affects bone remodeling. It turned out that this protein stimulates the production of a signaling substance called RANKL, which regulates bone turnover by creating a dialogue between bone-building cells called osteoblasts and bone-destroying cells called osteoclasts. In fact, when the scientists applied galectin-8 to osteoblasts, these cells produced six times more RANKL than usual. And when they applied galectin-8 to osteoblasts that were cultured together with bone marrow in a laboratory dish, many more osteoclasts than usual were produced from the marrow’s stem cells. Finally, the researchers examined their genetically engineered mice by computer tomography and discovered that despite normal appearance, the animals had unusually low bone mass and brittle bones.
However, the loss of bone mass was not due simply to increased numbers of osteoclasts. Rather, the mice had a higher than normal bone turnover: Their osteoblast levels were also elevated, but the osteoclast levels were elevated even further, so that the end result was excessive bone loss. This finding is significant because that is precisely what happens in human beings with osteoporosis, which in turn suggests that galectin-8 is involved in this disease in humans and can therefore serve as a potential target for the future development of drugs for treating it.
But the effect galectin-8 has on bone is not limited to osteoporosis. Recent studies in Zick’s lab have shown that this protein stimulates the activity of genes that may facilitate the spread of prostate cancer to the bone. A better understanding of this process may help prevent this metastasis.
Finally, in experiments conducted in a laboratory dish and animal models, Zick’s team found that in addition to stimulating the production of RANKL, galectin-8 causes osteoblasts to release a number of inflammatory molecules that cause blood sugar levels to rise abnormally. Indeed, their genetically engineered mice with elevated galectin-8 levels were found to have high blood sugar.
With this finding, research launched twenty years ago in Zick’s lab has come full circle. It began with studies of insulin resistance, which often leads to type 2 diabetes, and may now open up a new avenue of research into mechanisms leading to diabetes.
Apart from Dr. Vinik, taking part in these studies are Hadas Shatz-Azoulay, Dr. Alessia Vivanti, Dr. Navit Hever, Dr. Yifat Levy, Rotem Karmona and Dr. Sigalit Boura-Halfon – all current or former students in Prof. Zick’s lab – as well as Dr. Vlad Brumfeld from Weizmann’s Chemical Research Support. The study was a collaborative work with the group of Prof. Itai Bab from the Hebrew University of Jerusalem, who sadly recently passed away.
Lectins and Galectins
Weizmann Institute scientists have a long tradition of studying lectins and galectins. For several decades, starting in the early 1960s, Prof. Nathan Sharon conducted pioneering studies on the properties of lectins. In one of the projects, he and Prof. Yair Reisner, his former student, found that lectins could be used to isolate subpopulations of immune cells called lymphocytes. Reisner, together with physicians at the Memorial Sloan Kettering Cancer Center, subsequently showed that certain lymphocytes separated out with the help of a soybean lectin could enable mismatched bone marrow transplantation in “bubble children” suffering from severe combined immunodeficiency. It was another Weizmann Institute researcher, Prof. Vivian Teichberg, who in the mid-1970s, for the first time, found a galectin in an animal: an electric eel.
Prof. Yehiel Zick's research is supported by the Yad Abraham Research Center for Cancer Diagnostics and Therapy, which he heads; the Adelis Foundation; the estate of Fannie Sherr; and Ayala Benjamin-Mashat, Switzerland. Prof. Zick is the incumbent of the Marte R. Gomez Professorial Chair.