An energy supply is important for any undertaking; but in stem cells, energy-producing structures sometimes determine the very fate of the cell. A new Weizmann Institute study, reported in Nature Communications, reveals how cellular power plants called mitochondria can wake up blood-forming stem cells from their sleep, causing them to proliferate and mature into different cell types.
Blood-forming stem cells, which give rise to the entire immune system, lie sleeping in niches in the bone marrow. They are continuously woken up to replenish the blood with mature cells, which have a finite life span. The wake-up call can come in the form of reactive oxygen molecules called free radicals, which are produced in the mitochondria as a byproduct of the manufacture of cellular fuel. A team of Weizmann Institute scientists headed by
Prof. Atan Gross of the Biological Regulation Department has now discovered a mechanism by which the wake-up message is sent to these stem cells via their mitochondria.
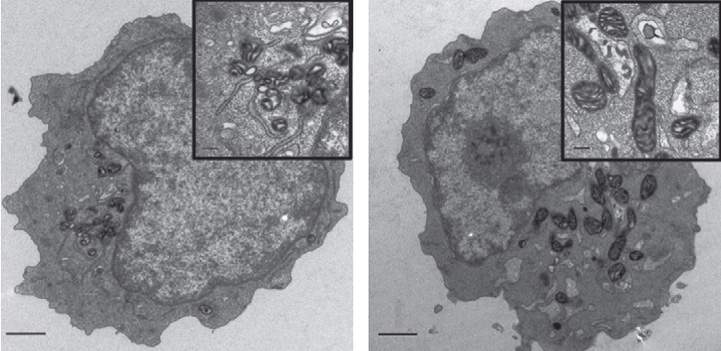
The heart of the message is a protein known as MTCH2 – or “Mitch,” as the scientists call it – which sits on the membranes of mitochondria and acts as a molecular switch. When Gross discovered MTCH2 more than a decade ago, he and his team showed that this protein can regulate cell suicide: Under conditions of severe stress, “Mitch” conveys a self-destruct message that prompts the mitochondria to develop holes and disintegrate, ultimately causing the cell to die. In the new study, postdoctoral fellow Dr. Maria Maryanovich and other members of Gross’s lab – Dr. Yehudit Zaltsman and PhD students Antonella Ruggiero and Andres Goldman – found that in blood-forming stem cells, MTCH2 has an additional role: It suppresses the activity of the mitochondria for as long as the cells need to remain in their dormant state.
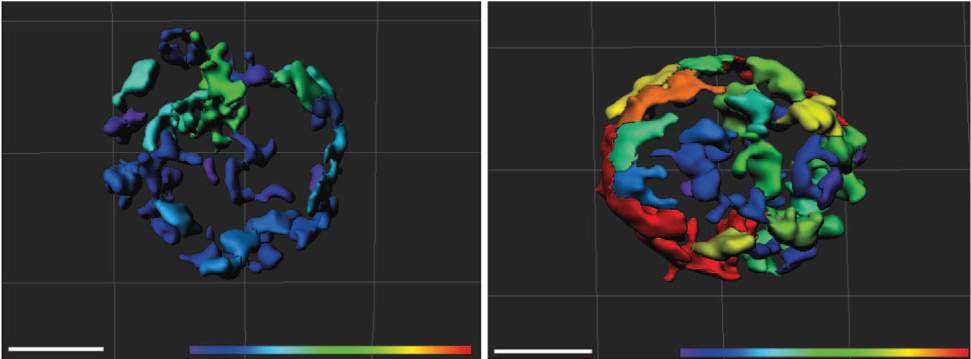
When the scientists created genetically engineered mice that lacked MTCH2 throughout their blood system, the mitochondria in the blood-forming stem cells underwent major changes. These organelles more than doubled in size, and their activity increased almost four-fold. As a result, the stem cells became activated, apparently woken from their sleep by the free radicals generated in the hyper-busy mitochondria. The cells left their niches and began to mature in such large numbers that their supply in the bone marrow was exhausted. These findings suggest that enhancing the activity of the mitochondria – by decreasing MTCH2 – can awaken the stem cells when needed.
This clever control mechanism of the stem cell cycle – awakening the cells by enhancing their metabolism – ensures that the cells have sufficient energy for growing and maturing. “Like travelers waking up in the morning and stocking up on essential provisions before undertaking a long journey, sleepy stem cells need the energy to survive their new journey after they awaken,” says Gross. “We found that turning on mitochondria metabolism supplies the cells with precisely such energy.” Taking part in the study were Dr. Smadar Levin Zaidman of Chemical Research Support, Dr. Ziv Porat of the Biological Services Unit, and Prof. Tsvee Lapidot and Dr. Karin Golan of the Immunology Department.
In addition to shedding new light on the basic biology of the stem cell cycle, the Weizmann Institute study may lead to new ways of controlling the activity of stem cells in research as well as in the clinic. The findings suggest that it may be possible to awaken stem cells by altering their metabolism, rather than by manipulating their genes, as is done today. In addition, the findings open up a new avenue of research into leukemia. They suggest that defects in the control of cellular metabolism in blood-forming stem cells at various stages of their maturation may lead to the abnormal cellular proliferation observed in leukemia. If this is indeed found to be the case, it may be possible to treat leukemia by correcting the cells’ metabolic defects.
Prof. Atan Gross’s research is supported by the Yeda-Sela Center for Basic Research; the Adelis Foundation; the Lubin-Schupf Fund for Women in Science; the Pearl Welinsky Merlo Foundation Scientific Progress Research Fund; the Louis and Fannie Tolz Collaborative Research Project; the Hymen T. Milgrom Trust donation fund; the Rising Tide Foundation; Lord David Alliance, CBE; the estate of Tony Bieber; and the estate of John Hunter. Prof Gross is the incumbent of the Marketa and Frederick Alexander Professorial Chair.


