עיתונאיות ועיתונאים, הירשמו כאן להודעות לעיתונות שלנו
הירשמו לניוזלטר החודשי שלנו:
Imagine taking a bulldozer that is normally used to demolish entire mountains and employing it to mold a fragile vessel. That’s just what living cells do. Ruinous enzymes called caspases, which often help cells to self-destruct, can sometimes serve to separate out or gently remodel cellular structures. How does the cell maneuver such lethal demolition agents without causing widespread damage?

A team led by Prof. Eli Arama of the Weizmann Institute’s Molecular Genetics Department investigated a delicate process: the separation of sperm cells from one another in developing fruit flies. In this process, caspases break down the cytoskeleton holding these cells together. The sperm are originally clumped in bundles; they become individuals as they split apart from the head to the tail. As they separate sperm cells from one another, the caspases also help destroy and remove superfluous surrounding structures, including the cytoplasm and most of the organelles, leaving only those structures that enable the sperm to reach its one and only goal: the egg.
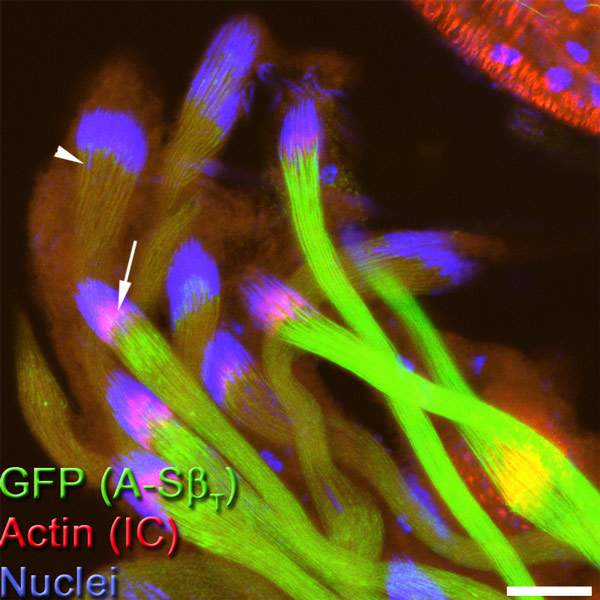
As reported in Developmental Cell, graduate student Lior Aram and other members of Arama’s team discovered that during sperm individualization, a molecular machine for activating the caspases – a member of a class of ubiquitin complexes – cannot independently unleash these enzymes. Rather, the complex must first bind to a protein called A-S-beta, which is present on the surface of two elongated mitochondria, the energy-producing organelles that supply the sperm cell with fuel for accomplishing its mission and which make up most of the sperm tail’s volume.
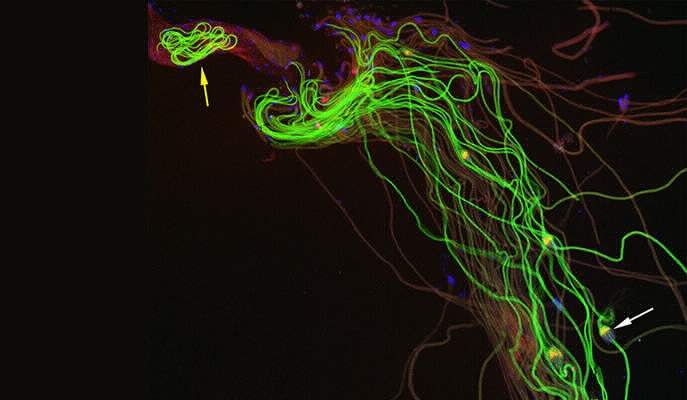
The binding to the A-S-beta is precisely what limits the rate of caspase activity: Only the ubiquitin complexes that have come into physical contact with this protein can activate caspases. The active caspases then move away from the vicinity of the mitochondria by diffusion, spreading throughout the cytoplasm so that their concentration is highest close to the mitochondria but drops gradually as the distance from the mitochondria increases. If it weren’t for this regulatory mechanism, the caspases would be activated all at once, killing the sperm or causing unwanted damage.
The fact that the caspase activation rate is regulated by A-S-beta, a protein that usually participates in the cell’s energy production inside the mitochondria (in a process called the Krebs cycle) probably ensures a quality control of sorts. The presence of A-S-beta on the surface of the sperm mitochondria, to where they have migrated from the organelles’ interior, may serve as an indicator that the cell has reached a required level in its energy metabolism and that everything about the sperm is in good order. In this manner, the A-S-beta makes sure the individualization of the sperm, their final maturation step, is launched at the proper stage and only for the properly functioning sperm.
Understanding how sperm individualization proceeds may shed new light on male infertility, which is sometimes caused by malformed sperm; but the study findings also have much broader implications. The details of caspase activation may provide new insights into the abnormal survival of cancer cells, which manage to evade the self-destruct commands dispatched by the caspases.
Moreover, apart from sperm individualization, the study findings may help unravel numerous other vital cellular processes, in which the caspases engage in limited destruction. Several dozen such processes are known. They include the formation of blood-clotting cells called platelets, the remodeling of neuronal synapses during learning, and the formation of the lens of the eye − rendered transparent through the activity of the caspases, which remove opaque structural elements. Future research may reveal whether A-S-beta or a similar protein helps prevent excessive caspase activation in these processes.
The research team included Tslil Braun, Carmel Braverman and Drs. Yosef Kaplan and Liat Ravid of the Molecular Genetics Department, and Dr. Smadar Levin-Zaidman of the Chemical Research Support Department.
Prof. Eli Arama's research is supported by the Yeda-Sela Center for Basic Research; and the late Rudolfine Steindling. Prof. Arama is the incumbent of the Harry Kay Professorial Chair of Cancer Research.