עיתונאיות ועיתונאים, הירשמו כאן להודעות לעיתונות שלנו
הירשמו לניוזלטר החודשי שלנו:

A number of weeks ago, I showed up to an almost vacant office in the Weizmann Institute of Science, holding a copy of my latest blood tests. I’m participating in a study called WizeAging, answering a call that has gone out for volunteers aged 50 and older. After filling out the usual form – do I smoke, drink, take any medication or drugs, have any diseases, etc. – Dr. Joseph Gardyn fills four test tubes with blood from my arm. Gardyn, a retired hematologist, is originally from Australia and he would have liked to chat with me, a fellow immigrant English-speaker, but other volunteers are waiting. I’m invited to come back once a year over the next decade to repeat the blood test.
So far, this sounds pretty low-tech. But the study, conducted by Dr. Liran Shlush and his group in the Immunology Department will lead, they hope, to the medicine of the future, in which blood tests will identify more diseases (for example cancer) long before symptoms appear. They are looking for genetic mutations in the blood that may predict any number of age-related diseases and help unravel their causes – hopefully leading to earlier diagnosis and treatment.
The results they will send me in about six months’ time will be just a small part of the story
The next week, I go to a cramped office in the basement of the Weizmann Institute of Science’s Britannia Building to get an explanation from Shlush and three members of his research group. They are reorganizing, they tell me, so that Gardyn can take the blood samples in their lab. Shlush explains that my blood is carefully aliquoted (divided up and conserved) to allow a large number of studies from the same test. Of the 40 cc taken, only about 400 microliters will be used for immediate sequencing analysis; the rest of the cells will be frozen viably for any future testing that may come up. The first thing done to each sample is to clear away all the red blood cells, leaving white blood cells, plasma and serum. This is because only the white blood cells contain DNA. Still, that leaves around 20 million cells per sample.

All of our blood cells – red and white – are continuously produced from blood stem cells that reside in the bone marrow. The stem cells divide, one daughter cell differentiating into a blood cell and the other remaining a stem cell. As we age, mutations can occur in the stem-cell DNA. Any cell in our body can acquire mutations, but when they occur in stem cells, each new daughter cell will contain the mutations. Every blood cell has roughly 1000 mutations, in total. The mutations Shlush and his team are looking for seem to occur mostly in certain locations on specific genes, and around 30% of subjects in the WizeAging study are likely to have at least one. “Some of the mutations may also give a stem cell an advantage, for example, enabling it to divide more frequently or survive better, so that more of the blood cells will contain these mutations,” explains Shlush. “This may lead to blood diseases and cancers. But other researchers showed several years ago that these stem-cell mutations also increase the individual’s chance of death from any cause – heart disease, diabetes and more. We want to know which mutations do what and why they affect us as we age.”
The members of Shlush’s group are breaking a bit of new ground at the Weizmann Institute with this study, since the Institute does not have a medical facility or clinic attached to it. (Shlush, however, is an MD/PhD, who treats leukemia patients.) Everything from taking blood to creating an anonymized database to analyzing the results is done within the lab, and this requires a great deal of organization and teamwork. Dr. Nathali Kaushansky, who doubles as the head staff scientist in Shlush’s lab, deals with everything from organizing blood tests and replying to emails to preparing the samples for the DNA sequencing. Dr. Yoni Moskovitz then takes the white blood cells and sequences their DNA in a method known as deep sequencing. This enables the scientists to precisely identify mistakes in some 80,000 nucleotides that are of interest, and to learn how often these mistakes appear. Dr. Noa Chapal Ilani works on the next stage of the study: the computational analysis. She is developing algorithms that look for the connections between certain mutations and heightened risk for various diseases. The group also conducts tests on the serum that can tell them, on the one hand, about changes in levels of hormones or other biochemicals that may tie in to disease, and on the other hand about mutations.
The chances that I could be carrying at least one of the blood stem cell mutations are considerably higher
So far, the group has sampled blood from nearly 150 volunteers, and they plan to get up to 500 this year. Eventually they hope to collect results from 10,000 people over the age of 50. I am coming to understand that the results they will send me in about six months’ time will be just a small part of the story. Shlush and his group are really looking for change. They not only want to know whether my blood and that of my fellow volunteers carries mutations now; they want to know if there will be new mutations in five or ten years, and what these can tell us about the ways in which we will each age – faster or slower, differently yet in similar ways. And while the chances of me carrying a mutation for the particular leukemia they are focusing on within the study are quite rare, the chances that I could be carrying at least one of the blood stem cell mutations are considerably higher. So at the very least, my results could prod me to go to the doctor for checkups a bit more often.
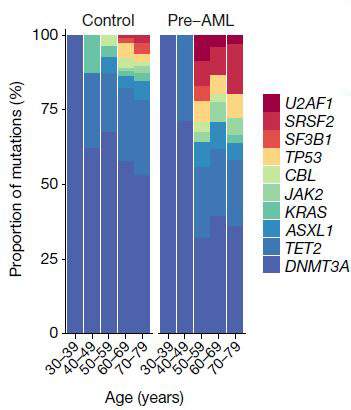
“If we see one particular set of mutations,” says Shlush, “we know with near certainty that the individual will develop AML – a rare form of age-related leukemia.” In a paper that was recently published in Nature, Dr. Sagi Abelson of the Princess Margaret Cancer Center, Toronto, Shlush and his group − George Vassilieu from the Sanger Institute in Cambridge, UK, and John Dick from the Princess Margaret Cancer Center − used information from a large European database, one in which around 500,000 people had had their blood cells’ DNA stored and had then been followed for 20 years. This enabled the researchers to work backwards, seeking to identify mutations that would have shown up in the DNA tests before the disease was diagnosed. They managed to pinpoint one set of early mutations in the AML patients, and observed that the cells carrying these mutations were the ones that multiplied more rapidly later on. From this, they determined a set of rules that could predict in the earlier tests, many years before the actual diagnosis, who would eventually be diagnosed with AML. This breakthrough could lead in the future to the early diagnosis and early treatment of AML. Shlush and colleagues from Memorial Sloan Kettering, NYC, and the Clalit research institute in Israel, are developing the first clinical trial for the prevention of AML, based on a specific mutation that affects the cells’ ability to make certain proteins.
It is currently impractical to have everyone over 50 undergo genetic testing for this rare disease. But Shlush, in collaboration with Prof. Amos Tanay of the Institute’s Computer Science and Applied Mathematics Department and Prof. Ran Balicer from Clalit, applied their insights to another database – that of the Israeli Clalit Health Services – and began developing an algorithm that would point to those people who would be at possible risk of the leukemia, just on the basis of their standard blood tests. This smaller group could then undergo DNA testing. “Now that we understand the problem, we want to develop treatments to eradicate just the cells with the mutations,” says Shlush.
“In the new study, we are bringing together all sorts of relevant information that was lacking in the two databases we used in the Nature study. In the future, we think that we might be able to identify the people who are at high risk for all sorts of diseases of aging,” he adds, “since many of these have an immune or inflammatory component driven by white blood cells. Ultimately we might then find ways to reduce their risk.”
Dr. Liran Shlush's research is supported by the David and Fela Shapell Family Foundation INCPM Fund for Preclinical Studies; the Steven B. Rubenstein Research Fund for Leukemia and Other Blood Disorders; the Morris and Ruth Wagner and Marek Sutkiewicz Laboratory for Cancer Research; the Felix and Silvia Schnur Endowment Fund in Stem Cell Research; Rising Tide; the Applebaum Foundation; Paul Goldensohn; the estate of Alice Schwarz-Gardos; and the European Research Council. Dr. Shlush is the incumbent of the Ruth and Louis Leland Career Development Chair.
Dr, Nathali Kaushansky is the incumbent of the Applebaum Foundation Research Fellow Chair.