In the late 1960s, three Weizmann Institute of Science researchers developed several protein-like molecules, called copolymers, that they believed would produce a disease similar to multiple sclerosis in laboratory animals. The scientists – Prof. Michael Sela, Prof. Ruth Arnon and Dr. Dvora Teitelbaum – were surprised to discover that, instead of causing the disease, the copolymers cured it; one of these molecules became the widely-used drug Copaxone. More than half a century later, in a new study being published today in Nature Cardiovascular Research, a research team from Weizmann’s Molecular Cell Biology Department, headed by Prof. Eldad Tzahor and Dr. Rachel Sarig, reveals that Copaxone might also facilitate recovery from a heart attack.

Heart attacks happen when the supply of blood to part of the heart muscle is cut off. Unless this supply is renewed quickly, the heart muscle cells start to die. Unlike skeletal muscle and other tissues that can recover from injury unscarred, heart muscle cells do not divide and do not replace dead cells with a new muscle. Instead, the heart’s fibroblasts (that is, fiber cells) divide rapidly in the damaged area and create a network of protein fibers that replace the damaged cells with scar tissue. This tissue ensures the integrity of the heart but reduces its ability to contract and pump blood. In the long term, therefore, a heart attack increases the chances of heart failure, a chronic condition in which the heart is incapable of meeting all the body’s needs, initially during physical exertion and later even while at rest. Heart failure affects around 64 million people around the world.
""Because the patent for Copaxone has expired, we are finding it hard to find partners in the pharmaceutical industry for continuing this research"
Over the past decade, the immune system’s response to heart damage has been shown to be directly involved in cardiac recovery and rehabilitation. But when the inflammation triggered by this response fails to subside and becomes chronic, the damage grows even worse and can lead to heart failure. Since it was already established that Copaxone alters the composition of cells in the immune system and the proteins they release, thereby suppressing inflammation, Sarig wondered whether it would be possible to use the drug to examine how the immune system influences recovery from a heart attack.
In the new study – led by Sarig and two research students from Tzahor’s lab, physician Dr. Gal Aviel and Jacob Elkahal – researchers treated mice that had suffered heart attacks with a daily abdominal injection of Copaxone. Echocardiograms revealed that the drug improved the functioning of the damaged mouse hearts and that their heart chambers were sending more blood to the large arteries with each heartbeat, which in turn supplied more of the vital blood to other organs. The scar area in the treated mice was relatively small; moreover, large scars covering at least 30 percent of the left chamber were observed only in mice that were not treated. People who suffer heart attacks do not always go to the emergency room right away, but the researchers discovered that Copaxone was effective in mice even when treatment began 24 to 48 hours after the heart attack.

The next stage of the study was to test the treatment in a rat model, but this time the researchers started treatment almost a month after the heart attack, when the rats already had chronic heart failure. By the end of the two-month treatment, the percentage of blood that was pumped out with each heartbeat climbed by an average 30 percent, and cardiac contractility – the ability of the ventricles to contract – improved by almost 60 percent. One month after the end of the treatment, the pumping of blood continued to improve and improvements in cardiac contractility persisted. So, while seeking to answer a fundamental scientific question – the extent to which the immune system affects heart rehabilitation – the scientists discovered a promising new possibility for treating a common heart disease.
To their surprise, the team also discovered that the drug works by not only influencing the composition of the immune system cells in the damaged area of the heart but also, apparently, by directly protecting the cardiac muscle cells themselves: Copaxone was found to protect heart muscle cells in tissue cultures that contained no immune cells. At a later stage, the treatment also halted the division of the fiber cells that form scar tissue and boosted the production of new blood vessels.
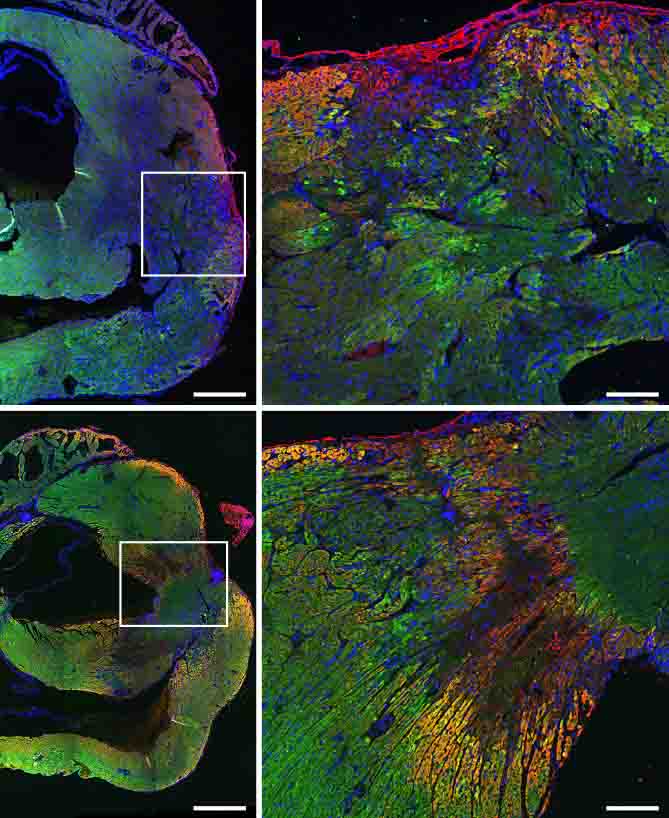
“Treatment with Copaxone does not cause heart muscle cells to divide,” Sarig explains. “It helps the existing cells to survive and contract effectively, enhances the production of blood vessels that supply them and delays the creation of scar tissue.” In light of their promising laboratory results, the Weizmann scientists, along with Aviel and other clinicians, joined forces with Prof. Offer Amir and Prof. Rabea Asleh from the Hadassah Medical Center in Jerusalem to conduct a phase 2a clinical trial examining the effectiveness of subcutaneous injections of Copaxone in patients with heart failure.
The results of this trial are yet to be published, but they are expected to show a rapid improvement in markers of both inflammation and heart damage. “Because the patent for Copaxone has expired, we are finding it hard to find partners in the pharmaceutical industry for continuing this research,” Tzahor says. “Still, repurposing an existing drug for a new use is quick and inexpensive compared to developing a new drug, and I hope that some donor or organization will pick up the gauntlet.”