Whenever a sink overflows, the flooding is usually caused by a blockage that has built up in the drains. Similarly, as we age our bodies are flooded by aging, or senescent, cells, which have stopped dividing but, instead of dying, remain active and build up in body tissues. Recent studies have shown that getting rid of these cells might delay age-related diseases, reduce inflammation and extend lives. Despite the great potential, however, there is currently no drug that can target these cells directly and efficiently.

Now, Weizmann Institute of Science researchers suggest an alternative approach. In a new study published in Nature Cell Biology, they reveal that senescent cells build up in the body by clogging up the immune system, thereby preventing their own removal. The scientists demonstrated in mice how to unclog this blockage using immunotherapy, the new generation of treatments that is revolutionizing cancer therapy. These findings could pave the way for innovative treatment of age-related diseases and other chronic disorders.
""While the treatment did not stop the aging clock, it did manage to get rid of senescent cells and even reduce the release of inflammatory proteins”
Prof. Valery Krizhanovsky’s lab in Weizmann’s Molecular Cell Biology Department has long been studying biological processes characteristic of aging, specifically, the involvement of senescent cells in age-related diseases and chronic inflammation. A mathematical model developed in 2019 by Weizmann’s Prof. Uri Alon, in collaboration with Krizhanovsky, predicted that while senescent cells are removed from a young body in a matter of days, in an aging body they manage to delay their own removal. The new study, led by Dr. Julia Majewska and Dr. Amit Agrawal, reveals the mechanism that makes this possible – how senescent cells evade the immune system the same way that cancer cells do.
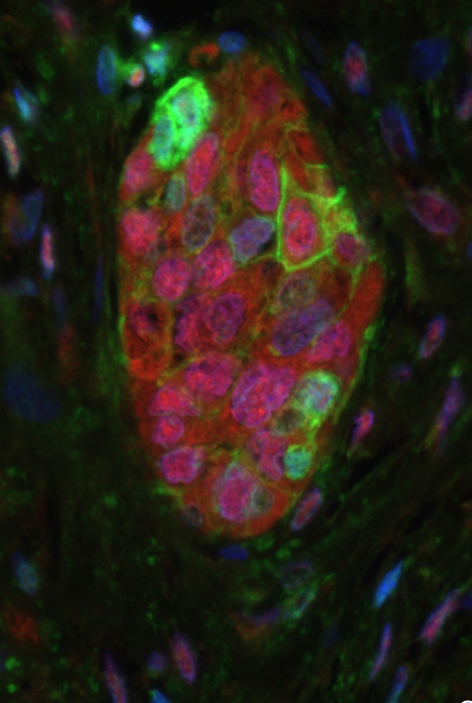
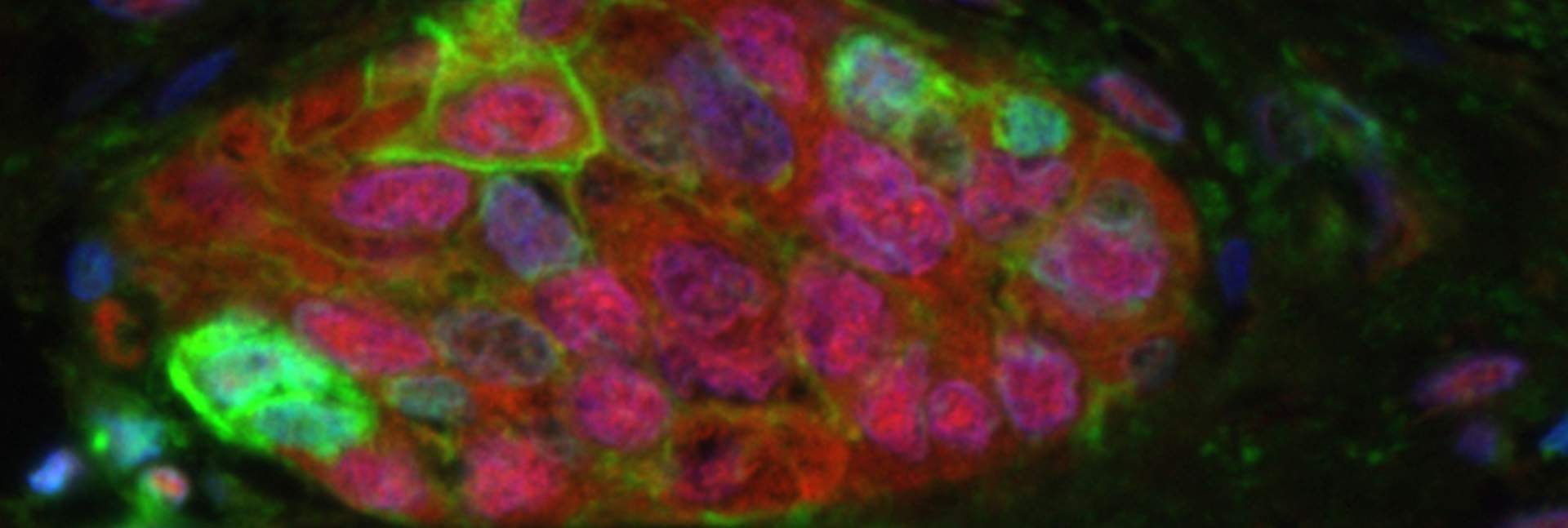
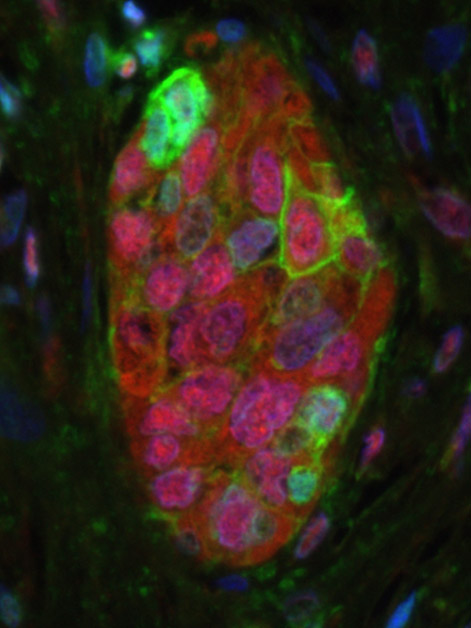
The researchers discovered that senescent cells from a mouse’s lung express high quantities of proteins that repress the immune system, particularly PD-L1. This protein, well known in oncology, is a key target for the development of new cancer drugs, since cancer cells have been shown to use PD-L1 to reduce the immune system’s ability to recognize and destroy them.
The question, however, was how this overexpression of an immune-suppressing protein comes about in the first place. The cellular aging process can be compared to pressing on the gas pedal and the brakes at the same time: Hitting the gas means that the cell remains highly active, while on the other hand, pressing on the brakes brings the cell to the end of its normal lifecycle and stops it from dividing. (Precisely for this reason, senescent cells are sometimes known as “zombies.”) A key component of the brakes is the p16 protein, which suppresses DNA replication in the cell. In their study the researchers discovered that there is a correlation between the rise in p16 during cellular aging and the increase in PD-L1 levels. They also worked out the molecular mechanism responsible for the increase: The p16 suppresses a natural cellular process that marks the PD-L1 to be broken down.

Beyond the senior moment
Senescent cells are, however, important not only in aging. In previous studies Krizhanovsky’s team had shown that the accumulation of these cells contributes to chronic lung diseases and other disorders. The current study shows how the levels of the PD-L1 protein increase not just during aging but also in a mouse model of chronic obstructive pulmonary disease, which is most common in smokers. Moreover, the researchers found that people with this disease have senescent cells that express elevated levels of p16 and PD-L1.
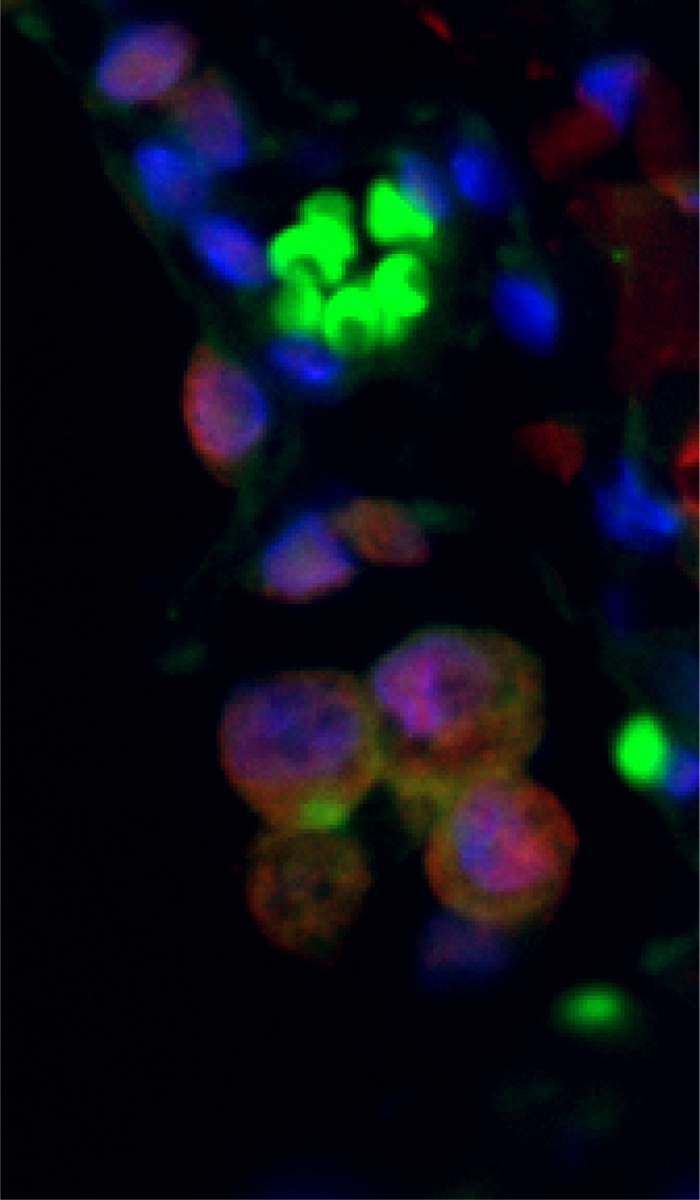
Once it became clear that senescent cells – like cancer cells – express high levels of PD-L1, which helps them evade the immune system, the researchers hypothesized that this knowledge could be used to target senescent cells with a relatively high degree of accuracy. They decided to take advantage of an antibody that has already been approved for treating various kinds of cancer, using it to identify PD-L1 in cellular membranes and activate the immune system against it. The researchers tested this antibody in aging mice, as well as in mice with short-term and chronic inflammatory damage to the lungs. As they expected, the antibody activated T cells – the immune system’s warriors – and other immune cells, which led to a reduction in the number of senescent cells.
“While the treatment we examined did not stop the aging clock, it did manage to get rid of senescent cells in mice and even reduce the release of small proteins that encourage inflammation in old age and in chronic diseases,” Krizhanovsky says. “Because PD-L1 is expressed in high quantities not only in senescent cells, we believe that the key to developing targeted, effective treatment will be engineering antibodies that can identify two proteins at the same time: PD-L1 and an indicator of aging. This discovery raises hopes that immunotherapy can be used in the future for treating not only cancer but also age-related diseases and chronic inflammation.”