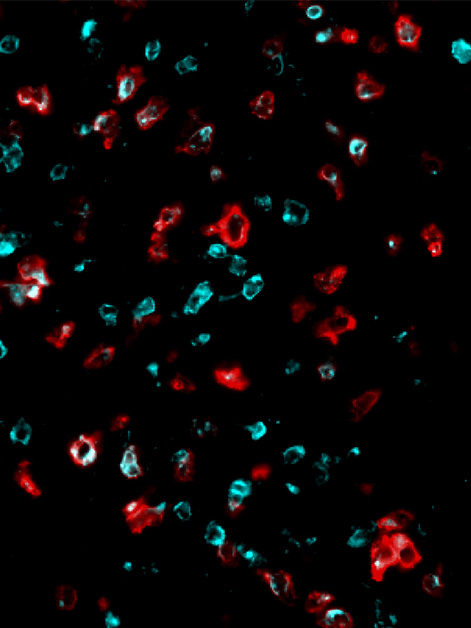
When your social media account starts spewing out nonsensical or threatening status updates, it’s safe to assume that it has been hacked and must be shut down. The cells in our body also update their “status” by presenting to their environment small proteins that are constantly being produced inside the cell. Our immune system monitors these statuses and destroys cells that produce unusual proteins. A classic example of this is when a cell is infected by a virus and presents parts of the viral proteins on its surface, which allows the immune system to recognize them and destroy the cell. In contrast, cancer cells often evade detection by displaying very few suspicious proteins that the immune system can identify and target. A new approach to cancer treatment, developed by researchers from Prof. Yardena Samuels’s lab at the Weizmann Institute of Science, increases the number of the immune system’s targets by disrupting protein production in cancerous cells. In a new study published in Cancer Cell, the researchers show that this disruption forces the cancer cells to expose themselves by producing dozens of suspicious proteins, which leads to a powerful immune response that is capable of destroying human cancer cells and slowing the development of aggressive tumors in mouse models.

Immunotherapy, the new generation of cancer treatments, enlists the patient’s immune system in battling tumors. Although immunotherapy has revolutionized cancer treatment, it is currently able to help only a minority of patients. To elicit an effective immune response, killer T cells – the immune cells responsible for identifying suspicious cells – first have to be able to identify the cancer cell as a foreign entity that must be eradicated. This can happen as a result of mutations in the protein synthesis recipes that lead to strange proteins being presented. However, many types of cancer harbor few mutations, leaving the immune system with very few effective targets for identifying and eradicating the cancer cells.
“The disrupted proteins don’t always result from a mistake in the DNA ‘recipe’ – that is, a mutation,” Prof. Samuels explains. “They can also stem from a fault in the production process itself, known as the translation. In our new study, we decided to investigate whether we could increase the number of targets for identifying and destroying cancer cells by intentionally interfering with the translation process.”
""Since the translation process is the same across cell types, a treatment that disrupts it in one cancer could work against many others"
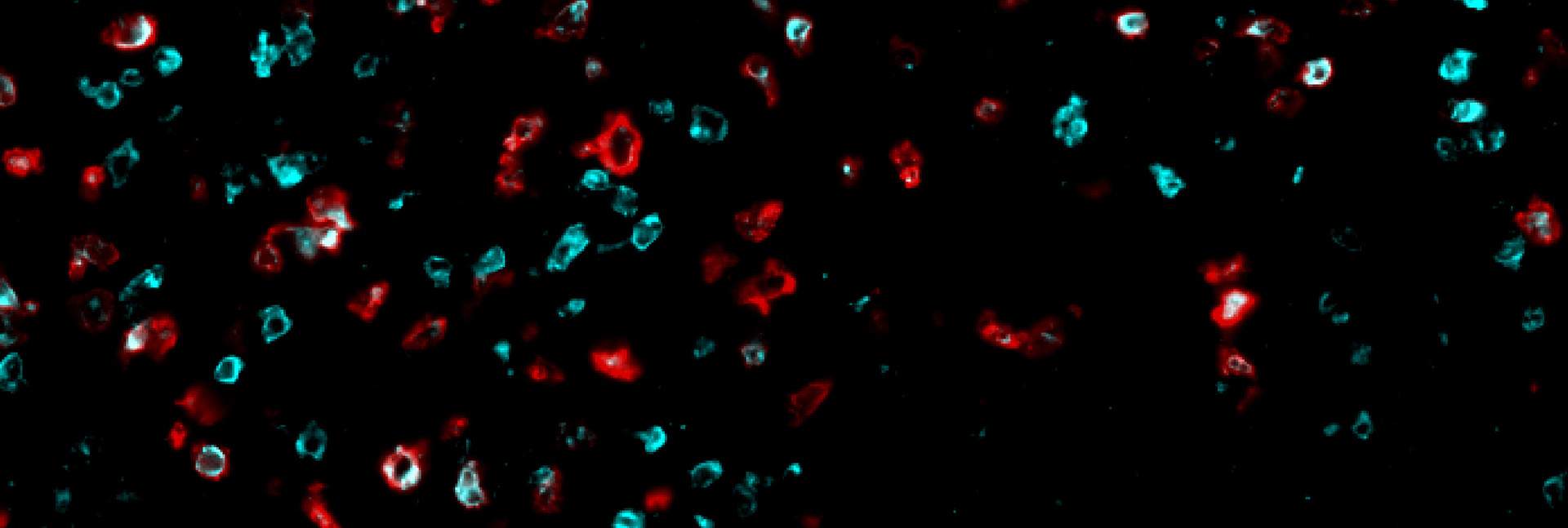
During the translation process, the ribosome, the cell’s protein synthesis factory, reads the genetic recipe and puts together a protein from its building blocks, amino acids. The recipe itself is a long sequence of RNA bases that function as “letters,” with every three letters representing a “word” saying which amino acid to add to the protein sequence. Since this is such a sensitive and vital process, many enzymes are involved in ensuring that the ribosome reads the genetic recipe accurately, without skipping any of the letters. To disrupt the translation process in human melanoma cells, the research team – led by Chen Weller and Dr. Osnat Bartok from Samuels’s lab and Dr. Christopher S. McGinnis from Stanford University – used genetic engineering to delete one of those enzymes. The deletion caused the ribosome to misread the genetic instructions, incorrectly divide the words and produce proteins with the wrong amino acids. In their study the researchers identified 34 short proteins, uniquely synthesized in cancer cells, that had been disrupted, and showed that some of them could be effective new targets for triggering an immune response against the cancer.
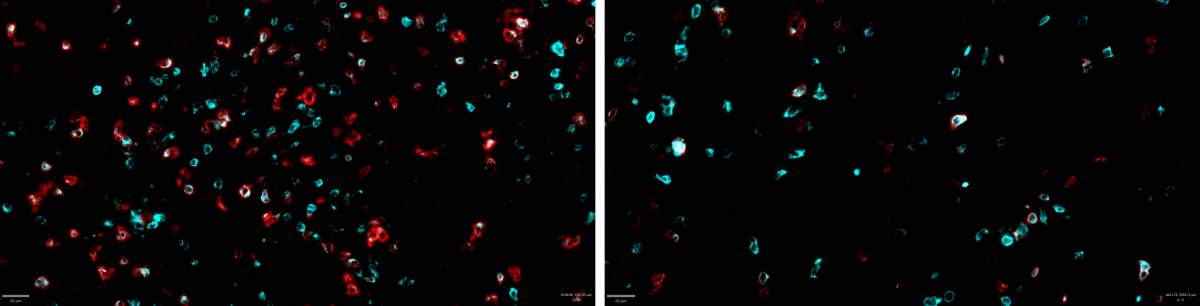
The next stage of the study was to examine, in a mouse model, whether disrupting the translation process in melanoma tumors would cause them to start triggering an effective immune response. When the researchers disrupted the translation and forced the cancer cells to synthesize aberrant proteins, the population of killer T cells that was activated to attack the tumor and penetrate its environment increased significantly. However, by the time the killer T cells reached the cancerous tumor, they were “exhausted” and no longer able to eradicate the cancer – a familiar problem in the field of immuno-oncology.
Some of the major current immunotherapy treatments were designed specifically to address this problem by blocking the immunosuppressive signals that the cancer sends to the T cells. The researchers hypothesized that, if they could combine the existing treatments with the new approach they had developed, the immune system would be able to fight tumors more effectively. “An existing type of immunotherapy that was not at all effective against the kind of melanoma we tested suddenly became very effective when tested in mouse models after the translation process in the mice’s cancer cells was disrupted,” Samuels explains. “This combined treatment managed to eradicate or greatly reduce the tumor in around 40 percent of the mice.”
A new standard for predicting the success of immunotherapy
These findings could lead to new cancer treatments in the future, but they could also have a more immediate impact. Currently, oncologists tend to prescribe immunotherapy to patients whose cancer has many mutations. There are, however, cancer patients who are not candidates for immunotherapy because their cancer has a small number of mutations, yet it’s possible that their tumors have low levels of the enzyme that ensures accurate translation. In their study, the researchers showed that such low levels can accurately predict the likely success of immunotherapy. “Finding a new predictive measure for the effectiveness of immunotherapy will allow doctors to offer the treatment to patients who, until now, were not candidates,” Samuels explains.
Apart from the clinical advances, the study also offers a completely new approach to treating cancer. “This is proof of feasibility, showing that deliberately disrupting the translation mechanism enhances the immune response against cancer cells,” Samuels says. “There are more than 600 different factors involved in the protein translation process, and they could be future targets for treatment development. In collaboration with Stanford University, we are already using AI tools to look for additional targets to disrupt in the cancer cell’s translation process. In addition, since the translation process is the same in different cell types, any treatment that successfully manages to disrupt this process in one type of cancer cell could be effective against many other types of cancer. We are already examining the possibility of disrupting the translation process in cancers of the breast and pancreas, as well as in colorectal cancer.”