עיתונאיות ועיתונאים, הירשמו כאן להודעות לעיתונות שלנו
הירשמו לניוזלטר החודשי שלנו:

Weizmann Institute scientists have uncovered a key mechanism leading to the spread of cancerous colon cells and have succeeded in reversing their metastasis in laboratory tests. The study, published in the Journal of Cell Biology, raises hopes that target-specific drugs might be devised to prevent, or reverse, the metastatic stage of colon cancer.
The second most prevalent type of cancer in men and the third in women in the Western world, colon cancer is lethal largely because its cells spread to a variety of other tissues, primarily the liver.
Normal cell growth and repair are orchestrated through an intricate checks-and-balances system controlled by a circuitry of genes and their respective proteins. Tumor formation is generally triggered by a mutation in one or several of these genes, causing the circuitry to go off track.
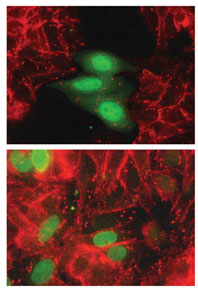
The team of Institute researchers headed by Prof. Avri Ben-Ze’ev of the Molecular Cell Biology Department has now traced one of the key cellular circuitries that, when mutated, leads to metastatic cancer of the colon. Its main players are two “cell-gluing” molecules known as beta-catenin and E-cadherin, and a cancer-causing gene called Slug. The team showed that the malfunction of these adhesion-related molecules causes the cancer cells to break loose from the tissue and migrate to form another tumor at a distant site.
They found that abnormally high levels of beta-catenin (due to a mutation in the beta-catenin gene itself or in one of the genes controlling its breakdown) cause a surge in Slug, which then inhibits the production of beta-catenin's partner in cell adhesion, E-cadherin. The resulting E-cadherin shortage prevents the cell from adhering to adjacent cells. “The cell takes on a boat-like shape and, leaving the pack, enters the bloodstream and migrates to distant colon tissue, where it proceeds to multiply, forming a new tumor,” explains Ben-Ze’ev.
The scientists found that when colon cancer cells are surrounded by other such cells in the crowded environment created in a test tube, minute quantities of E-cadherin recruit beta-catenin from the nucleus and bind to it. Lower levels of beta-catenin in the nucleus then result in decreased Slug production, leading, in turn, to increased E-cadherin production. As a result, the cells stick together and form a tissue-like organization, losing their invasive properties. This finding parallels a recent study in colon cancer patients by pathologists at Erlangen University in Germany. In examining lymph node tumors that had spread from the patient’s colon, the researchers found that some of the cells naturally maintained their invasive character, while others reordered into a crowded, normal “tissue-like” organization. Something about their new environment induced cell repair. This is precisely the process that Ben Ze’ev’s team hopes to promote to block metastasis. The question is how to tilt the scales in favor of the formation of normal tissue.
“The fact that the invasive process in colon cancer can be turned around is surprising,” says Ben-Ze’ev. “It offers hope of reversing the metastatic process, or even preventing it, in the future. One idea would be to design a drug that would raise the levels of the adhesive E-cadherin protein by targeting its inhibitor - Slug.”
Prof. Ben-Ze’ev’s research is supported by the M.D. Moross Institute for Cancer Research; the Yad Abraham Center for Cancer Diagnostics and Therapy; the estate of Maria Zondek; and La Fondation Raphael et Regina Levy. He is the incumbent of the Samuel Lunenfeld-Reuben Kunin Professorial Chair of Genetics.