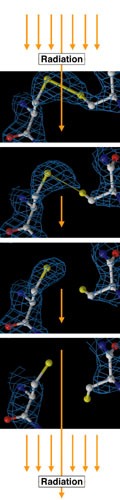
Weizmann Institute researchers recently got more than they'd bargained for. While studying an enzyme pivotal to brain function and memory, they changed an experimental focus and ended up capturing the first-ever time-resolved "movie" demonstrating how molecules break apart when exposed to X-rays.
The team discovered that the "flash" they were using to study the chemical reaction of the acetylcholinesterase (AChE) enzyme was essentially destroying their target. Since the reaction takes place within microseconds, the researchers had planned to record the process by taking an extremely rapid series of X-ray "snapshots." But upon close examination, they discovered that instead of capturing the enzymatic reaction, they had actually obtained the first-ever 3-D recording of how chemical bonds break apart when exposed to radiation.
"The observation was stunning," says Prof. Joel Sussman of the Weizmann Institute's Structural Biology Department. "The time-series movie looks like a simulated animation of chemical processes; but in fact it's the real thing - a direct experimental observation that has never been made before." Sussman conducted the research together with Drs. Gitay Kryger and Michal Harel of the Structural Biology Department and Prof. Israel Silman of the Neurobiology Department. Their findings were published in the January issue of the Proceedings of the National Academy of Sciences (PNAS).
Subsequent studies revealed that, contrary to previous belief, radiation damage affects specific, weak parts of protein structures. These parts include the disulfide bonds that often bridge protein polypeptide chains, and carboxyl acids found at the "active site" where enzymatic reactions start. The scientists also found a cross-species similarity, suggesting a more general phenomenon; results were similar whether working with AChE crystals derived from torpedo fish, humans, the Drosophila fruit fly, or even an entirely different enzyme - the hen egg white lysozyme.
These findings have direct implications for improving the X-ray crystallography techniques used to study biological molecules. It's all about balance. While X-rays are key to viewing microscopic worlds, they also cause radiation damage, often destroying the experimental sample. The crystallographic community has traditionally walked a thin experimental line, increasing X-ray intensity to get more information, while cutting radiation damage through cryo-crystallography (data collection at extremely cold temperatures).
"One of the most important take-home lessons is that less intensive radiation may provide more accurate results," says Kryger. "The key is to avoid introducing inadvertent changes into experimental samples, such as those induced by radiation damage."
The ability to visualize at test-tube level the specific damage caused by radiation also offers an important tool for developing pharmacological measures to protect against high-dose radiation - a common cause of cancer and birth defects. Organisms are constantly exposed to radiation, whether from natural sources, such as sunlight and cosmic rays, or man-made sources. The Weizmann team and its European collaborators plan to examine the antiradiation potential of various substances that could be applied on a conventional basis or in an emergency such as that which followed the Chernobyl nuclear power plant failure.
"In science, it is quite common to find answers to one question when seeking answers to an entirely different one. While looking down one avenue we were essent- ially sidetracked into an alley, with perhaps even broader applications," says Kryger.
The Weizmann team worked in close collaboration with Martin Weik, Maria Raves, Piet Gros, and Jan Kroon - all from Holland's Bijvoet Center for Biomolecular Research at Utrecht, as well as with Raimond Ravelli and Sean McSweeney of the European Molecular Biology Laboratory Outstation at Grenoble, France.
Prof. Israel Silman holds the Bernstein-Mason Chair of Neurochemistry. The research of Profs. Silman and Sussman is supported by the Charles Dana Foundation, New York.